할로카본 계열 소화약제를 이용한 소화 (Extinguishment with Halocarbon agents)
(출처: NFPA Fire protection handbook. 20th edition)
▣ 이산화탄소 소화약제와의 비교
<이점>
Halogenated agents have the following two principal advantages over carbon dioxde:
- Certain halogenated agents are effective in low volumetric concentrations such that sufficient oxygen remains in the air after compartment flooding for comfortable breathing.
- For several halogenated agents, only partial vaporization occurs initially during flow from a nozzle, allowing the liquid to be projected farther than carbon dioxide.
정리:
- 특정 할로겐화 소화약제는 구획실 내에 약제 방사 후 호흡이 불편하지 않을 정도로 충분한 공기가 남아 있을 만큼 소량의 약제만 방사되어도 소화에 효과가 있다. (질식소화 메커니즘 X)
- 여러 할로겐화 소화약제의 경우 노즐로부터 방사시 부분적인 기화만 이루어지고 이 때문에 이산화탄소 소화약제보다 더 멀리 액체 상태로 방사가 가능하다.
<결점>
The drawbacks of using even the new generation of halocarbon agents relate to agent toxicity and the toxicty and corrosivity of agent decomposition products during a fire.
Of the first-generation halocarbons, Halon 1301 (bromotrifluoromethane) was by far the most commonly used in fire protection because it has the lowest toxicity as well as the highest effectiveness on a weight basis, including the highest volatility, which is desirable for flooding applications. If a halocarbon liquid was needed for direct application to a burning surface to accomplish cooling as well as inerting of the nearby region, however, a less volatile compound, such as Halon 1211 or Halon 2402 was the first-generation agent preferred.
정리:
- 할로카본 소화약제 자체의 독성과 연소 과정 중의 분해생성물의 독성과 부식성이 있다.
- 1세대 할로카본 소화약제 중에는 Halon 1301이 가장 기화가 잘되는 특성을 가지고 있으며 중량대비 가장 적은 독성과 가장 뛰어난 소화성능을 가지고 있다.
- 1세대 할로카본 소화약제 중 연소 중인 물질 주변의 불활성화뿐만 아니라 연소 중인 물질의 직접적인 냉각효과를 원하는 경우 상대적으로 기화성이 적은 Halon 1211 또는 Halon 2402가 선호됨.
▣ 물리적 특성 및 불활성화 능력
Table (A) gives the physical properties of these three halocarbons. They are all liquids at normal temperatures when stored in pressurized tanks. They can be sotred under high-pressure nitrogen if the liquid must be expelled from the tank more rapidly than under the vapor pressure of the halon alone. The use of nitrogen for pressurization is especially important for outdoor storage in the winter.
[Table (A)]: Physical Properties and Chemical Formulas of three Halon Extinguishing Agents.
| Property | Halon 1301 (CF3Br) | Halon 1211 (CF2ClBr) | Halon 2402 (C2F4Br2) |
| Boling Point [℃] | -58.00 | -4.00 | +47.00 |
| Liquid density at 68℉ (20℃) [g/cc] | 1.57 | 1.83 | 2.17 |
| Latent heat of vaporization [J/g] | 117.00 | 134.00 | 105.00 |
| Vapor pressure at 68℉ (20℃) [atm] | 14.50 | 2.50 | 0.46 |
The inerting capabilities of Halon 1301 and Halon 1211 are shown in Table (B). If methane in any proportion is combined with a mixture containing 5.4 volumes of Halon 1301 and 100 volumes of air, at 77 ℉ (25 ℃), then no combustion can result. By contrast, a mixture containing 33 volumes of carbon dioxide and 100 volumes of air is required to obtain the same result. This suggents that a molecule of Halon 1301 is 6.1 (33/5.4) times as effective as a molecule of carbon dioxide. Note, however, that the molecular weight of Halon 1301 is 149, whereas that of carbon dioxide is 44, so the ratio of molecular weights is 3.39(149/44). Accordingly, on a weight basis, Halon 1301 is only 1.8 (6.1/3.39) times as effective as carbon dioxide for methane fire.
정리:
- 메탄의 경우 대기 중 할론 1301이 공기대비 5.4:100 (할론 1301 : 공기 부피비) 이상 존재할 경우 연소가 진행되지 않음. 이산화탄소 소화약제가 동일한 효과를 얻기 위해선 33:100 이상 필요.
- 즉, 할론 1301의 소화약제 분자의 소화성능은 이산화탄소 보다 6.1배 더 효과적이다.
- 하지만 소화약제의 무게까지 고려했을 때 할론 1301의 소화효과는 이산화탄소 소화약제보다 1.8배 더 효과적이라고 할 수 있다.
[Table (B)]: Minimum required volume ratios of Halons to Air at 77 ℉ (25 ℃) that will prevent burning of Various vapors
| Vapor | Halon 1301 | Halon 1211 | ||
| 1301 / air | % O2 | 1211 / air | % O2 | |
| Hydrogen (H2) | 0.290 | 16.2 | 0.430 | 14.7 |
| Carbon disulfide (CS2) | 0.150 | 18.2 | - | - |
| Ethylene | 0.130 | 18.5 | 0.114 | 18.8 |
| Propane | 0.073 | 19.5 | 0.065 | 19.7 |
| n-Hexane | - | - | 0.064 | 19.7 |
| Ethyl ether | 0.070 | 19.6 | - | - |
| Acetone | 0.059 | 19.8 | 0.054 | 19.9 |
| Methane | 0.054 | 19.9 | 0.062 | 19.7 |
| Benzene | 0.046 | 20.0 | 0.052 | 19.9 |
| Ethanol | 0.045 | 20.0 | - | - |
Table (B) also shows that the addition of Halon 1301 or 1211 needed in the air will only reduce the oxygen percentage from 21 percent to about 19 percent for most combustibles, whereas the required amount of carbon dioxide would have reduced the oxygen level to 14 percent or 15 percent.
정리:
대부분의 가연물을 대상으로 소화효과를 위해 요구되는 할론 1301이나 1211의 약제량은 산소농도를 21%에서 19% 정도로만 낮추는 것에 불과하지만 이산화탄소 소화약제의 경우 필요한 소화효과를 보기 위해선 공기 중 산소농도를 14~15%까지 낮출 만큼의 이산화탄소가 요구됨.
▣ 할론 1301(CF3Br) 과 할론 1211(CF2ClBr)의 효과성
Graph (A) shows a methane-air flammability limit diagram as modified by various volumetric proportions of bromotrifluoromethane or carbon dioxide. The enormous difference is obvious. It has been established that carbon dioxide acts by absorbing heat and reducing the flame temperature from about 3452 ℉ (1900 ℃) for a stoichiometric fuel-air mixture to about 2192 to 2372 ℉ (1200 to 1300 ℃). Below these temperatures, most flames can no longer burn.
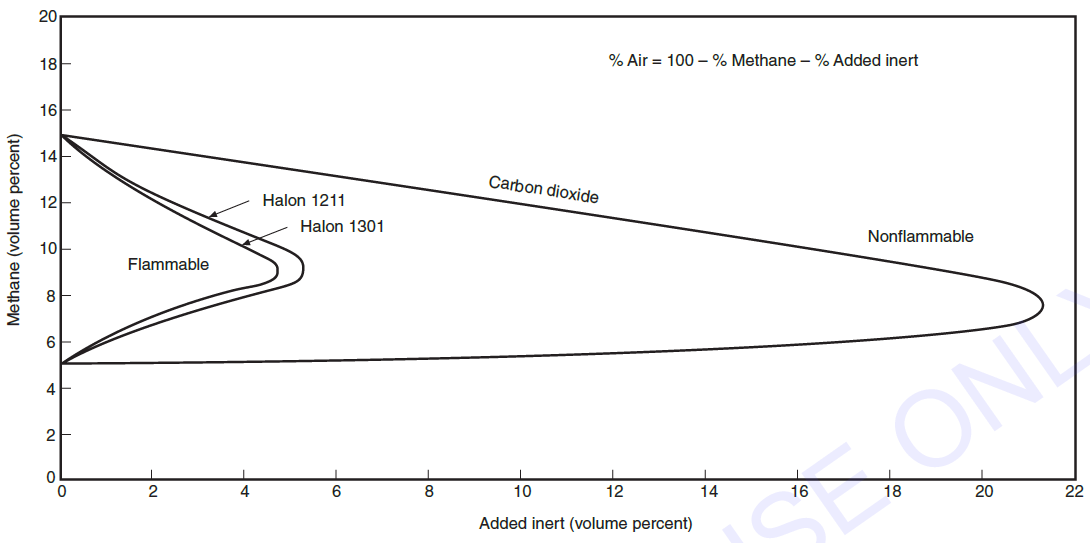
If nitrogen were added to a stoichiometric fuel-air mixture instead of carbon dioxide, a somewhat larger volume of inert agent would be needed because the heat capacity of the nitrogen molecule is less than that of the carbon dioxide molecule. Similarly, if argon were added - argon has an even lower heat capacity per molecule- an even larger volume of argon would be needed for inerting. In each case, the flame would go out when the temperature dropped below 2192 to 2372 ℉ (1200 to 1300 ℃). However, if a small volume of bromotrifluoromethane were added to a flame, so that the temperature dropped to only about 2732 ℉ (1500 ℃), the flame would be extinguished. Clearly, the mechanism is different.
정리:
- 화학양론농도의 조건을 갖추고 연소 중인 화염에 이산화탄소, 질소, 아르곤 등의 불활성 기체를 첨가할 경우 화염의 온도는 1,900℃에서 1,200 ~ 1,300℃까지 온도가 떨어지며 이 온도 이하에서 대부분의 화염은 연소가 지속되지 않는다.
- 화염의 온도를 1,200 ~ 1,300 ℃까지 낮추는데 필요한 불활성 가스의 부피는 이산화탄소 < 질소 < 아르곤이다. (열용량(Molar heat capacity) 차이)
- 하지만 할론 1301(CF3Br)의 경우 소량을 방출하여 화염의 온도가 1,500 ℃ 정도까지만 떨어져도 연소가 종료된다. (이산화탄소의 소화메커니즘과 다름)
The important chemical reactions in flames involve the free atoms H and O and the free radical OH, which undergo chain reactions with the fuel and oxygen. In particular, the branching chain reaction H + O2 → OH + O is very important. It is believed that the CF3Br molecule decomposes in the flame to form HBr, and HBr then acts to remove H atoms and OH radicals by the following two combustion reactions:
HBr + H → H2 + Br
and
HBr + OH → H2O + Br
HF and HCl cannot react as rapidly with H or OH as can HBr, so bromine appears to be essential to the inerting molecule. It has been found that hydrogen iodide, HI, is about as effective as HBr, but iodine is more expensive and heavier than bromine as well as quite toxic, and so iodine offers no advantage over bromine for flame extinguishment. In addition to the destruction of chain carriers, it has been speculated that a secondary contribution of halons to flame extinguishment comes from the extreme sootiness of halogen-containing flames.
The role of fluorine in halocarbon agents is twefold. First, fluorine atoms replace hydrogen atoms in methane or ethane, thereby reducing the flammability of the inerting agent itself. Second, the toxicity of the agent is reduced. For example, CH3Br is much more toxic than CF3Br , and, again, CH2ClBr is much more toxic than CF2ClBr.
정리:
- 할론 1301 (CF3Br)의 성분 중 Br이 연쇄반응을 차단하는데 중요한 물질.
- CF3Br이 연소과정 중 분해되며 생성된 HBr은 활성라디컬 OH, H와 결합하여 활성라디컬을 제거.
- 반면 분해과정 중 생성되는 HF, HCl은 활성라디컬 OH, H와의 결합반응이 HBr만큼 빠르지 않음.
- F(불소; fluorine)의 역할은 크게 두 가지로 ① 메탄, 에탄 등의 수소원자를 치환하여 가연성을 줄여주고, ② 독성을 줄여줌.
'HSE > 소방공부' 카테고리의 다른 글
| 할로겐화합물 및 불활성기체 소화약제의 인체에 대한 유해성 (LOAEL, NOAEL) 과 PBPK (Physiologically based pharmacokinetic) 모델링 (0) | 2023.11.10 |
|---|---|
| 구획실 화재의 연소속도(질량감소율)와 연소지속시간 영향인자 (3) | 2023.05.16 |
| 일반/고층 건축물과 위험물 제조소 등의 소화전 방수량, 방수압, 수원 등 비교 (3) | 2023.05.15 |